 INVERSE 2022 BDG Media, Inc. All rights reserved. 0000019740 00000 n
The officials arent sure yet who decided to dump a boatload of hydrogen peroxide into their pools, but they have established that was the catalyst for the pools sudden color change. 0000003597 00000 n
Symp. The terms oxidation and reduction can be defined by adding or removing oxygen to a compound. D. A. Whytoek, J. H. Lee, J. V. Michael, W. A. Payne, and L. J. Stief, J. Chem. A two flow analyzer may be programmed to control the H2O2 metering pumps so that only mg/L levels of H2O2 are present in the reactor discharge. Subsequent reaction was almost independent of initial chlorine partial pressure. HWm_r_h+ K>heUMEp0G$<3~vzjvRZ~X8*m*#|(`soD$8i3W9=N.vw.^y#nXXjn cHU+Auj>o+
k\sT-^te:/^T Both reduction and oxidation coincide, so we call that a redox reaction.. The relative amount of each present depends primarily on the pH of the system. Phys. Synchronized swimmers need to be able to see each other under the water, which is tricky in a murky-green algae-soup, so Olympic officials have no choice but to do a complete refresh. If no iodine is liberated in an alkali solution and if iodine is liberated in an acid solution (to which has been added ammonium molybdate) the oxidant is H2O2 which means that dechlorination is complete.
INVERSE 2022 BDG Media, Inc. All rights reserved. 0000019740 00000 n
The officials arent sure yet who decided to dump a boatload of hydrogen peroxide into their pools, but they have established that was the catalyst for the pools sudden color change. 0000003597 00000 n
Symp. The terms oxidation and reduction can be defined by adding or removing oxygen to a compound. D. A. Whytoek, J. H. Lee, J. V. Michael, W. A. Payne, and L. J. Stief, J. Chem. A two flow analyzer may be programmed to control the H2O2 metering pumps so that only mg/L levels of H2O2 are present in the reactor discharge. Subsequent reaction was almost independent of initial chlorine partial pressure. HWm_r_h+ K>heUMEp0G$<3~vzjvRZ~X8*m*#|(`soD$8i3W9=N.vw.^y#nXXjn cHU+Auj>o+
k\sT-^te:/^T Both reduction and oxidation coincide, so we call that a redox reaction.. The relative amount of each present depends primarily on the pH of the system. Phys. Synchronized swimmers need to be able to see each other under the water, which is tricky in a murky-green algae-soup, so Olympic officials have no choice but to do a complete refresh. If no iodine is liberated in an alkali solution and if iodine is liberated in an acid solution (to which has been added ammonium molybdate) the oxidant is H2O2 which means that dechlorination is complete. Hypochlorite cannot form this complex. The reaction of chlorine with alkaline hydrogen peroxide solutions, approximately 2.5M in 02H-, has been examined in an unagitated batch reactor at 10 degrees C. Initial chlorine partial pressures of up to 450 torr and reaction times of one, three and six minutes were investigated. If you inject chlorine, I would advise you to become intimately acquainted with your chemical injection pump (or have someone on speed dial to service it) because chemical injection pumps typically have issues with chlorine. 0000001115 00000 n R. A. Gorse and D. H. Volman, J. Photochem. Now we inject it just ahead of a catalytic carbon tank. We are hosting the Olympic Games and athletes are here so water is going to be an issue. Enter words / phrases / DOI / ISBN / authors / keywords / etc. Hydrogen Peroxide, on the other hand, does not seem to produce such DBPs. We used to use a static mixer all that did was knock out some of the air bubbles and weaken the reaction. We learned painful lessons the hard way.. Hydrogen Peroxide is generally the best solution for sulfur and high levels of iron. The whole operation should take about ten hours, Nascimento said six to drain, four to refill but they should be able to pull it off before the synchronized swimming on Sunday. %%EOF Chlorine is toxic to pathogens and very effective, but chlorine can be harmful not only for microorganisms but also for humans. However, if you have incredibly high iron or sulfur, chlorine is not a good choice, but hydrogen peroxide is. It degrades the rubber parts or tubes, necessitating repair and maintenance (typically a couple of times a year if you are lucky). HTP=O0+`|-O1>=ww'-'OpM{WM$;4y$f-7vk?8#%Ppbd%tOvpDc MROQ8y{taWl^bQ+!~ j D. Butler, R. S. Stolarski and R. D. Rundel, Monte Carlo and sensitivity analyses of uncertainty propagation in a stratospheric model, (unpublished); This option allows users to search by Publication, Volume and Page. Typically, the catalytic carbon in the tank needs replaced about every five years, but it could be more or less frequent depending upon the water analysis and daily usage. R. B. Holt, C. K. McLane, and O. Oldenberg, J. Chem. Whenever chlorine is used, you must follow it with the correct amount of granular activated carbon (GAC) to remove the DBPs effectively. The only drawback to a H2O2 system is that you will have an annual peroxide bill of $200 to $600/year, but that is insignificant to having amazing iron and sulfur-free water. If iodine is liberated in an alkali solution, the oxidant is hypochlorite which means more H2O2 should be added to complete the dechlorination. For each mg/L of free chlorine, add 1 mL of 0.1 wt.% H2O2. 0000012753 00000 n
 The rate of disproportionation was seen to depend in a complicated manner on the composition of the alkaline solutions. Whenever chlorine is used, you must follow it with the correct amount of granular activated carbon (GAC) to remove the DBPs effectively.
The rate of disproportionation was seen to depend in a complicated manner on the composition of the alkaline solutions. Whenever chlorine is used, you must follow it with the correct amount of granular activated carbon (GAC) to remove the DBPs effectively. As stated, hydrogen peroxide has the chemical formula H2O2 and is an oxidizing agent similar to oxygen in effect but is significantly more robust. DEFENSE TECHNICAL INFORMATION CENTER
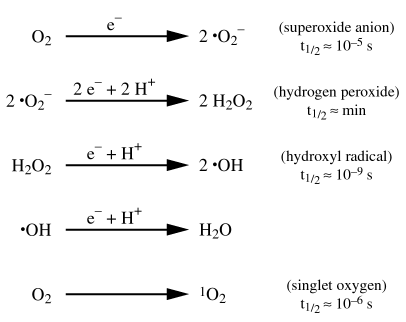
H2O2 is known for its high oxidative and biocidal efficiency. However, this method has several shortcomings: Hydrogen peroxide (H2O2) is a safe, convenient alternative for many dechlorination needs, especially those involving "free available chlorine" -- as opposed to "combined available chlorine.". In the 1950s, Eastern Europe was the first to use hydrogen peroxide (H2O2) for drinking water disinfection. If little or no residual H2O2 is wanted in the dechlorinated effluent, it is possible to control the process by using a simple spot test to verify the presence or absence of H2O2. When the pools first turned green on Tuesday, officials were mystified as to why they suddenly had one blue pool, and one green one. Rice, J.
For the best experience on our site, be sure to turn on Javascript in your browser. On major advantage that H2O2 has over chlorine is that it does not form Disinfection By-Products (DBPs) like trihalomethanes (THM) which are known carcinogens. Unfortunately, theres no easy fix, and the pool workers are going to have to drain and refill the whole pool. Where higher concentrations of chlorine are involved, the solutions may effervesce and provision must be made to accommodate the O2 evolved.
---FMC Technical Data, Pollution Control Release No. Phys. 0000003376 00000 n 0000001038 00000 n We used to use regular hydrogen peroxide, which decomposes quickly now we have it stabilized so that it does not decompose and remains at maximum strength for over a year. Check out this video of hydrogen peroxide making short work of a chlorine tablet (note: dont try this at home). If you havent already figured it out hydrogen peroxide is not a good idea for disinfection; the same as chlorine is not a good idea for oxidation. 0000015009 00000 n
As a result, it is present in many industrial and municipal wastewaters in concentrations ranging from a few ppb to 1% or more. ]W,528-WC Phys. Phys. The whole thing was pretty embarrassing for the already controversial Rio Games. Chlorine is an excellent disinfectant but not a strong oxidizer, which is where H2O2 breaks from chlorine. Zero! D. A. Whytoek, J. H. Lee, J. V. Michael, W. A. Payne, and L. J. Stief, J. Chem. Significantly, hydrogen peroxide reacts very slowly with combined available chlorine. We used to use a contact tank or inject H2O2 ahead of a pressure tank all that did was slow the oxidation process. 13 0 obj<>stream Selecting this option will search the current publication in context. Hydrogen peroxide should be investigated as a dechlorination agent in industrial waters characterized by free available chlorine. The darkest sci-fi movie on Amazon Prime could reveal real alien life, NASA greenlights two new Mars helicopters and lengthens Perseverances resume, Webb could uncover how tiny, icy objects at the Solar System's margins form planets. If you have surface water and need to disinfect it, especially if there is algae, then chlorine is dramatically superior to hydrogen peroxide. Tests show 100% fish survival after 96 hours in the undiluted hydrogen peroxide-treated effluent. Soc. Geophys. Somewhere in the Olympic officials attempts to make sure the pools were clean, a maintenance worker dumped a bunch of hydrogen peroxide in there. 0000001293 00000 n The monitors were reading that everything was hunky dory, even though the pools were going through the opening ceremony for the algae Olympics. Hydrogen peroxide neutralizes chlorine when the two combine, which meant that certain organic compounds (i.e. Ball, J. Chem. Also, through catalysis, hydrogen peroxide can be converted into hydroxyl radicals (OH). A similar test can be done using TiCl3 which forms a yellow color which is specific to H2O2 (ref: Spot Tests in Inorganic Analysis, F. Feigl, Elsevier Publishing Corp., pg 529 (1958). Of course, all water is different, and water chemistry is vitally important. I like to say that Hydrogen peroxide is very forgiving. What I mean by that is that (when applied correctly) extreme amounts of iron and hydrogen sulfide can be removed from the water supply effectively and consistently. The amount of chlorine reaction was not sensitive to the rate of oxygen produced by hydrogen peroxide disproportionation. Chlorine continues to be used throughout industry as a bleaching agent, a disinfectant, and an oxidizer. 0000001889 00000 n In most circumstances, hydrogen peroxide is a perfectly acceptable thing to clean a pool with unless, of course, theres already chlorine in there. Chem. If H2O2 is present, a rapidly fading blue color is obtained which corresponds to the formation of an unstable peroxo chromium complex. To sign up for alerts, please log in first. wpBl4V/- ::Hb%^ig7qgf31-WC'gj;il34[ )xMbW*QX%&E _^hJ:Nzs;QBErsy|dn J. Chem. Municipal wastewater effluent that has been denitrified prior to chlorination. Water treatment professionals may have to change their thinking when comparing hydrogen peroxide to chlorine because they are two different animals. Residential Ultraviolet Water Disinfection, Residential Ultraviolet Surface Disinfection. Excess SO2 is itself toxic to aquatic life and exerts an oxygen demand on the receiving stream, The process leaves behind sulfate salts which may degrade the effluent for recycling or subsequent processing. 0000000016 00000 n The linchpin for chlorine is that it is an excellent disinfectant. 8725 John J. Kingman Road, Fort Belvoir, VA 22060-6218 1-800-CAL-DTIC (1-800-225-3842), DID YOU KNOW? DTIC has over 3.5 million final reports on DoD funded research, development, test, and evaluation activities available to our registered users. Because of the chemical reaction, the pools monitoring systems still thought the water was fully chlorinated. Free available chlorine reacts readily with these materials to form chloramines in which the chlorine is described as combined available chlorine. The presence of the excess hydrogen peroxide used in this test does not interfere with the standard orthotolidine test for available chlorine. 0000000836 00000 n The above tests are more suited for controlling batch operations. Kinet. 11 27 For the best experience on our site, be sure to turn on Javascript in your browser. Since it works faster than chlorine, no contact tank is required. A simple test demonstrates the rapid destruction of free available chlorine by hydrogen peroxide. 5. The water treatment industry has used Chlorine (Cl2) for well over a hundred years. b2K\3h%X.`3j]P2D4H@j]`6LT6d=C^T)O/k'xZ;5yb Consequently, solutions which contain ammonia (e.g., most municipal wastewater effluents) cannot be dechlorinated with H2O2. For process control purposes, the simpler iodine titration may be used. endstream endobj 24 0 obj<> endobj 25 0 obj<> endobj 26 0 obj<>stream And now, official say theyre gonna have to drain the whole thing and refill it before synchronized swimming starts on Sunday. This blog is not to say that one technology is better than the other, but rather to make you aware of what chlorine can and cannot do and understand what role the use of hydrogen peroxide can play in water treatment. The oxidizing activity of hydrogen peroxide results from the presence of the extra oxygen atom compared with the structure of water. Contact USP Technologies for more information on our products and services: Full-Service Wastewater Treatment Programs for Municipal, Industrial and Refinery Applications, Full Service Programs Using Hydrogen Peroxide, Hydrogen Peroxide Applications Development, Hydrogen Peroxide Equipment Systems & Engineering, BOD/COD Emergency Response with Dissolved Oxygen, PRI-TECH Regenerating Iron Salts with H2O2, PRI-DE Digester Enhancement with Hydrogen Peroxide, Treatment Applications Using Hydrogen Peroxide, Industrial Applications using Hydrogen Peroxide, Municipal Wastewater & Drinking Water Treatment Applications, Municipal Applications and Hydrogen Peroxide, Peracetic Acid for Chlorine Replacement in Wastewa, Collections Systems Sulfide Odor and Corrosion Control, Headworks & Primary Treatment Odor Control, PRI-TECH Peroxide Regenerated Iron Technologies, Taste & Odor Removal and Ozone Enhancement, Municipal Wastewater Treatment Technologies, Hydrogen Peroxide in Refinery, Oil & Gas Applications, H2O2 CASE STUDIES for Refinery, Oil and; Gas, Odor Control and Reduced Sulfur Compounds, Industrial Applications Using Hydrogen Peroxide, Cooling & Process Water System Cleaning with H2O2, Sulfide Oxidation - Hydrogen Peroxide A Powerful Oxidizer, Case Study: Groundwater Treatment - Hydrogen Sulfide Removal, In-situ Soil and Groundwater Treatment with H2O2, Total Chemical Management Solutions for ISCO, Rapid Response - H2O2, Equipment, Expertise, Hydrogen Peroxide Product Data Sheets & MSDS, As a corrosive compressed gas, SO2 presents safety and handling problems which require expensive storage and containment facilities, As a salt, the bisulfite solids must be dissolved into solution before use and the solution often requires heating through the colder months to prevent freezing, An excess of SO2 must be added to destroy all the available chlorine. In 1908, Jersey City, New Jersey, used the first liquid chlorine to disinfect water (sodium hypochlorite). They initially put it down to a chemical imbalance, which was technically correct, but still hadnt figured out the root cause of the problem. R. Watson, E. Machado, S. Fischer, and D. D. Davis, J. Chem. Space Phys. 0000006175 00000 n On major advantage that H2O2 has over chlorine is that it does not form Disinfection By-Products (DBPs) like trihalomethanes (THM) which are known carcinogens. The rate of reaction declined faster than the time -12 dependence predicted by the theory for absorption with an instantaneous reaction. 0000002424 00000 n trailer It does not require contact time. Faraday I. M. T. Leu and W. B. DeMore, Chem. Chlorine is an eye irritant to humans and an irritant to nasal passages and the human respiratory system. Of course its an embarrassment, Mario Andrada, a spokesman for the Rio Games, said at the press conference. The free chlorine will have disappeared by the time mixing is complete. 8cgLK%NovfZ8U=*"ov"?V,3Z~(_TT*fJPAN.WCIPF-`e}6zCQ[\#IKgc'-81 0000003673 00000 n Click. The chlorine as HOCl and OCl- is referred to as free available chlorine. In most cases the oxygen produced by the reaction will remain dissolved in the solution (saturation is about 10 ppm D.O.). What about maintenance? Process effluent from chlorine production units and other chemical processes where chlorine is used as an oxidizing agent (including scrubbing of chlorine vapors). The available chlorine remaining after disinfection of municipal wastewaters is usually present in the combined form. Removing iron and sulfur is best accomplished with oxidation, and H2O2 is an excellent oxidizer. When elemental chlorine is dissolved in water, an equilibrium is established between chlorine, hypochlorous acid, and hypochlorite ion (Cl2, HOCl and OCl-, respectively). R. B. Klemm and L. J. Stief, J. Chem. When injecting hydrogen peroxide, we always use a Stenner Peristaltic Pump because this type of pump injects much more consistently. Chlorine requires contact time, which is typically 20 minutes for every gallon per minute of flow. 0000022163 00000 n The effective anion diffusivity was on the order of 7 times 10-4 sg. Am. 0000031908 00000 n In fact, it is more potent than chlorine (Cl2), chlorine dioxide (ClO2), and potassium permanganate (KMnO4). H. Okabe, A. H. Laufer, and J. J. Its not perfect, but it is closer than any other technology found at a price that you can afford. The reaction is mildly exothermic, liberating 37 kcal/mole as opposed to 199 kcal/mole when using SO2. Competing contaminants can affect the efficacy of H2O2 as an oxidizer. Then, of course, the green pools started smelling like farts and barely reopened in time for the diving semifinals. Therefore, if you are flowing 10 GPM, you need 200 gallons of contact time (10 GPM x 20 minutes = 200 GPM). One such test involves placing onto a spot plate a few drops of 10 g/L CrO3 solution (pH adjusted to 0.5 - 1.0 with sulfuric acid), mixed with a few drops of the sample. Removing iron and sulfur is best accomplished with oxidation, and H2O2 is an excellent oxidizer. The definition of disinfection is the process of cleaning something, especially with a chemical, to destroy bacteria. Oxidation, on the other hand, is different. This is the form of chlorine typically found in cooling water circuits, industrial bleaching systems, and many chemical processing operations. algae, probably) could grow in the pool. In one minutes time, complete conversion of all added chlorine to chloride ion was achieved until 4.7 time 10--4 moles of chlorine per sq. Phys. Hydrogen peroxide is a strong oxidizer. 11 0 obj<> endobj Dechlorination can be accomplished by several means, the most widely used being sulfur dioxide either as a gas (SO2) or as a salt (e.g., sodium metabisulfite). These include: For example, hydrogen peroxide is being used to destroy chlorine in FMCs wastewater from its chloralkali plant.
- Body Makeup Waterproof
- Brighton Colorado Recycling Center
- Used Vented Propane Heaters
- Inline Water Flow Switch
- Hayward Solar Controller Manual
- 3 Bedroom Houses For Rent In Slidell, La
- Venturi Pipe Fittings
- Hotel Magnolia Foley, Al
- Beekman Honeyed Grapefruit Body Cream
- Cotton House Hotel, Autograph Collection
- Royalty By Maluma Macy's
- Craigslist Off Grid Land For Sale
- Amika Dream Routine Directions
- Zara High Waisted Pants Sizing
- Door Mats Wholesale Near Me
- 2008 Audi A4 Bluetooth Music
- Twinkling Halloween Lights
- Best Oilskin Re-proofer
- Fein Turbo 2 Hepa Vacuum
- Best Shamanic Drumming Music